To evaluate the effectiveness of BIOintestil®, a real-life, open-label study was conducted in collaboration with Italian gastroenterologists on patients experiencing symptoms consistent with Irritable Bowel Syndrome (IBS).
IBS is currently the most common functional gastrointestinal disorder. It is a chronic and relapsing condition that, in the absence of identifiable organic causes, is diagnosed based on characteristic symptoms such as
- altered gut motility,
- visceral hypersensitivity,
- abdominal bloating,
- low-grade intestinal inflammation and
- gut dysbiosis.
You can learn more about this condition reading our article “Irritable Bowel Syndrome: Symptoms, Causes, and Insights”.
Today, symptom relief remains the primary therapeutic goal in IBS management. Treatment is tailored to each patient’s clinical profile and typically involves a multidisciplinary approach. While conventional over-the-counter medications are commonly used for their accessibility, safety, and low cost, they may be limited by side effects, contraindications, and suboptimal long-term outcomes.
In recent years, lifestyle interventions have gained recognition as effective first-line strategies in the management of IBS. Among these are dietary changes and increased physical activity, along with complementary approaches such as phytotherapy.
The Innovation Roadmap Behind the Real-Life Study
Current research has not yet clarified whether IBS-related dysbiosis is a cause or a consequence of intestinal inflammation. However, it is widely accepted that dysbiosis plays a key role in the pathophysiology of the disorder and likely contributes to the persistence of symptoms.
Essential oils have attracted growing interest in the management of gastrointestinal disorders due to their ability to modulate inflammation, oxidative stress, immune activation, and microbial imbalance. Among them, palmarosa essential oil (Cymbopogon martinii), which is particularly rich in geraniol, has shown promising pharmacological properties, including
- selective antimicrobial,
- anti-inflammatory and
- antioxidant activity.
In an in vivo model, oral administration of a low-absorbable geraniol formulation reduced intestinal wall Cyclooxygenase-2 (COX-2) expression, improved colitis, and favorably modulated gut dysbiosis. In a subsequent pilot study on IBS patients, the same formulation reduced scores on the IBS-Visual Analog Scale (IBS-VAS) and led to significant improvements in quality of life.
BIOintestil® was developed to optimize the delivery of palmarosa essential oil to the gut microbiota. Thanks to its very-low-absorbable formulation, the systemic bioavailability of the essential oil -specifically geraniol – is reduced from 92% (free form) to just 16%, allowing approximately 85% of the active compound to reach the colon intact.
From Controlled Trial to Real-World Evidence
The efficacy of BIOintestil was initially demonstrated in a double-blind, randomized, placebo-controlled clinical trial involving 56 IBS patients. The treatment led to a significant reduction in symptom severity—assessed through the IBS Severity Scoring System (IBS-SSS)—and produced beneficial changes in gut microbiota composition.
To confirm these findings under everyday conditions, a broader real-life open-label study was launched, involving a larger population of IBS patients.
Experimentation keypoints
The study was an interventional multicenter clinical trial conducted in an open-label manner with a dietary supplement based on BIOintestil® (Eurekol®, distributed in Italy by Diadema Farmaceutici s.r.l.). The study population consisted of patients diagnosed with IBS, enrolled by 31 private Italian gastroenterologists. Each patient in the study was administered a food supplement based on BIOintestil® for 30 days.
The severity of IBS was calculated using the validated IBS-SSS questionnaire. All details regarding timelines, study design, IBS-SSS Questionnaire scoring, and inclusion/exclusion criteria are provided in the infographic below.
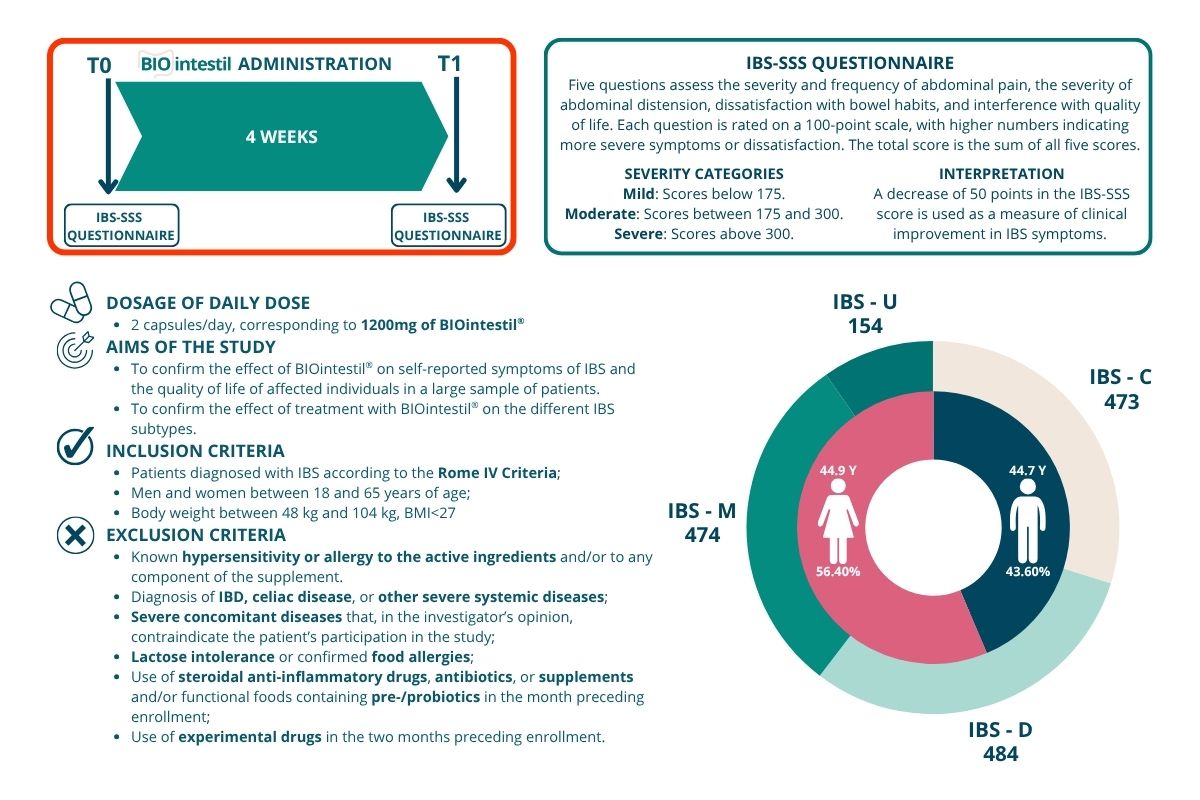
Figure 1 – The infographic illustrates the study design, the dosage of the formulation used, the study objectives, and the inclusion and exclusion criteria applied for enrollment. It also includes a description of the IBS-SSS questionnaire.
Results of the Real-Life Study
Statistical analyses identified significant differences in the participants’ symptomatology between V1 and V2. During the treatment with BIOintestil®, the average symptomatology score of the disease changed positively from moderate to mild severity.
Figures below demonstrate a highly significant reduction, with p-value < 0.01, in the total score after the 30 days of treatment, with the mean value dropping from 238.66 ± 82.91 to 76.69 ± 65.35 (Figure 2). The trial confirmed the same highly significant result (p < 0.01) in each IBS subtype (Figures 3 to 6). In particular, patients with IBS-U reported the greatest benefits.
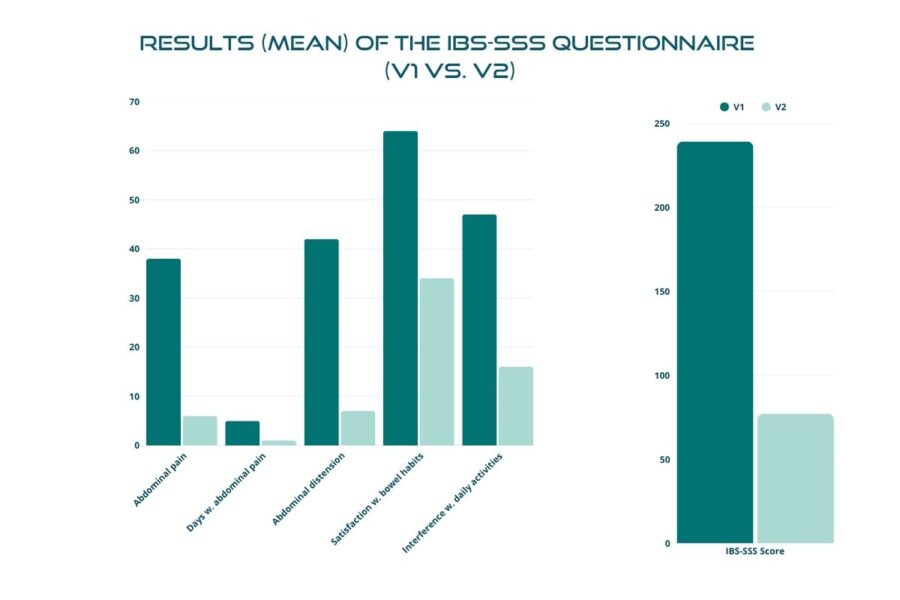
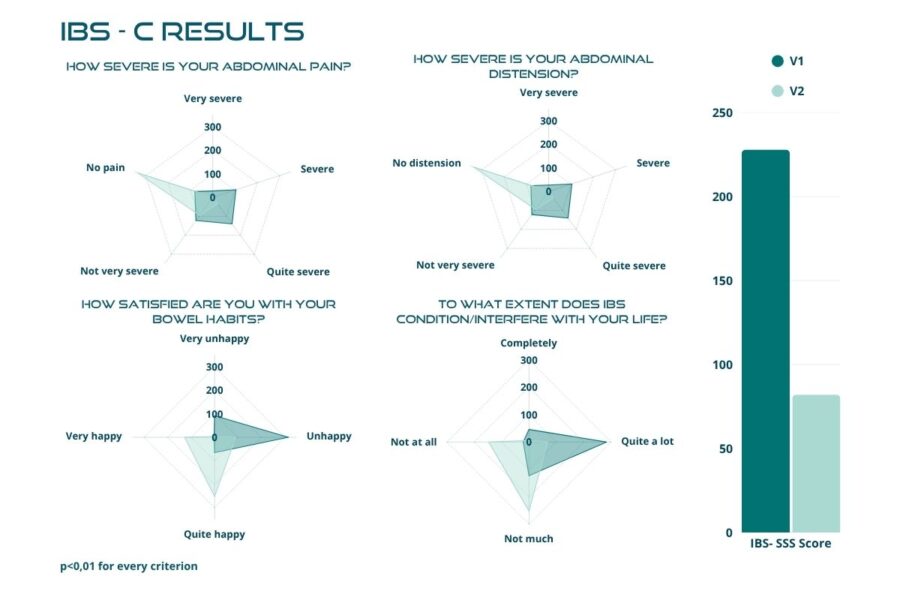
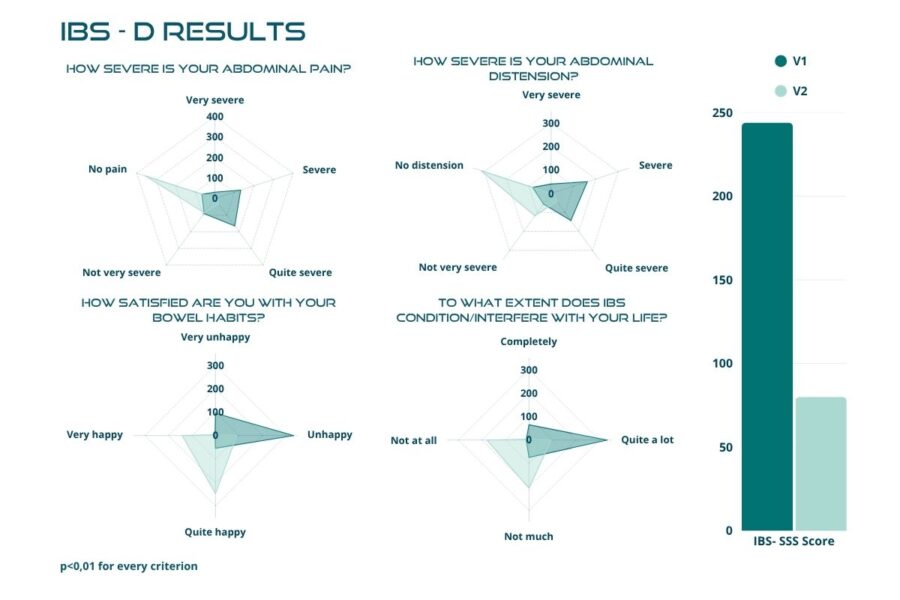
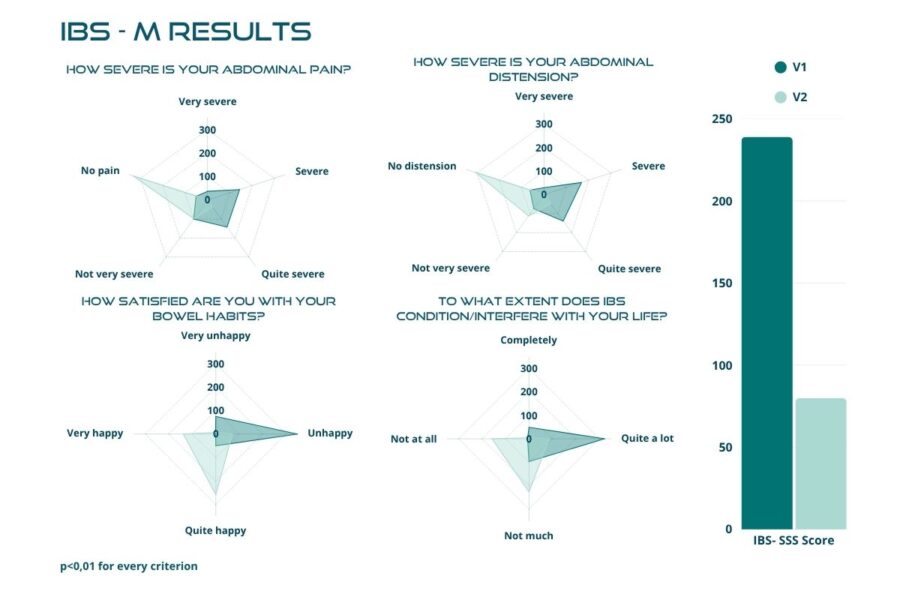
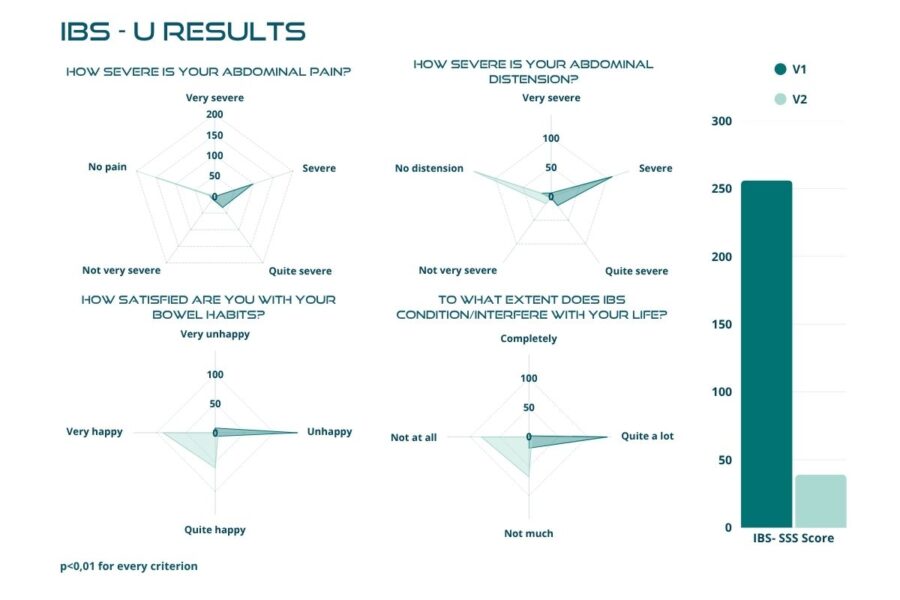
These data must be interpreted considering the open-label experimental design and the absence of a placebo arm. Considering these factors, we can reasonably consider a placebo effect of approximately 35%. In this scenario, the actual efficacy of BIOintestil® could be estimated at about 40% of responders. Overall, the observed improvement in IBS symptomatology and QoL associated with the pharmacological effects of BIOintestil® are in line with results from our previously reported clinical trials.
Questi dati devono essere interpretati considerando il disegno sperimentale in aperto e l’assenza di un braccio placebo. Tenendo conto di questi fattori, possiamo ragionevolmente considerare un effetto placebo di circa il 35%. In questo scenario, l’efficacia reale di BIOintestil® potrebbe essere stimata intorno al 40% di responder. Complessivamente, il miglioramento osservato nella sintomatologia dell’IBS e nella qualità della vita, associato agli effetti di BIOintestil®, è coerente con i risultati dei nostri precedenti studi clinici.
Bibliography
Ricci, C.; Saracino, I.M.; Valerii, M.C.; Spigarelli, R.; Bellocchio, I.; Spisni, E. Very-Low-Absorbable Geraniol for the Treatment of Irritable Bowel Syndrome: A “Real-World” Open-Label Study on 1585 Patients. Nutrients 2025, 17, 328. https://doi.org/10.3390/nu17020328
